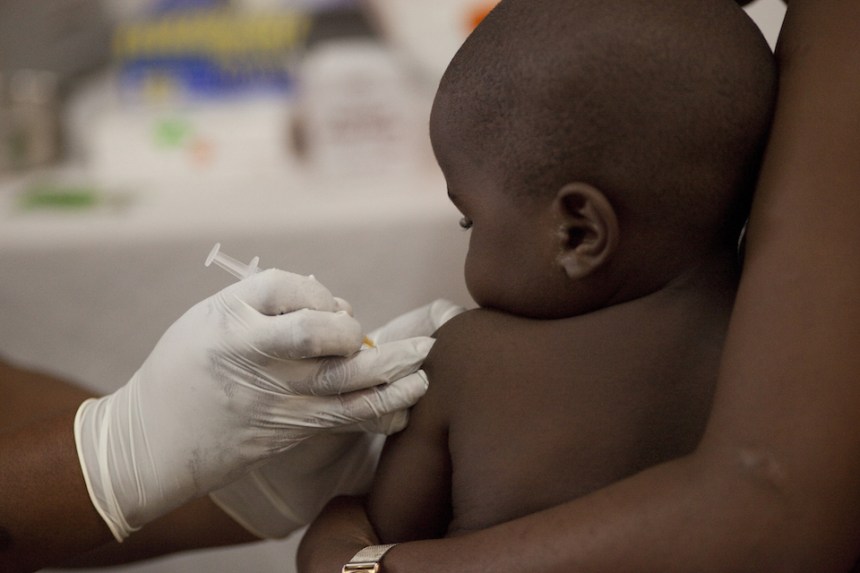
The Africa Centres for Disease Control and Prevention (Africa CDC) has approved the use of artemether lumefantrine, a malaria treatment, for infants weighing less than five kilograms, a breakthrough expected to save millions of young lives across Africa.
Clinical trials conducted in eight African countries, Burkina Faso, Côte d’Ivoire, Kenya, Malawi, Mozambique, Nigeria, Tanzania, and Uganda, showed that the drug is both safe and effective for infants in this weight category.
Until now, no malaria drug had been specifically developed for these children, forcing health workers to rely on medicines intended for older children, a practice that sometimes led to dangerous underdosing or overdosing.
The new formulation is designed to be mixed with breast milk and contains mild flavors, making it easier for infants to take without spitting it out because of bitterness.
It was developed through a partnership between pharmaceutical company Novartis and Medicines for Malaria Venture (MMV), under the oversight of the PAMAfrica consortium.
Swiss regulators were the first to approve the drug, and Africa CDC expects health authorities in the eight participating countries to follow suit soon.
In addition, Novartis has pledged to provide the medicine free of charge to children as part of a broader effort to reduce malaria deaths.
According to Africa CDC, around 30 million children are born each year in malaria endemic countries, the vast majority in Sub Saharan Africa.
Malaria remains one of the leading killers of children and pregnant women in the region.
The disease is caused by the plasmodium parasite, transmitted by the anopheles mosquito, which thrives in tropical climates.
Dr. Jean Kaseya, Director of Africa CDC, welcomed the breakthrough, calling the new drug a vital step toward giving African children a healthier start in life.